

Historical Tales | News | Vampires | Zombies | Werewolves
Virtual Academy | Weapons | Links | Forum
 |
 |
Historical Tales | News | Vampires | Zombies | Werewolves Virtual Academy | Weapons | Links | Forum |
Note from Dr. Pecos: Robert Lomax has written an excellent overview of vampire science that provides a more in-depth analysis than was previously available on this site. This is the first part; more to follow.
To view the abridged version of this section, click here.
Vampiric Virology
In 1616, Italian scientist Ludovico Fatinelli published his Treatise on Vampires, in which he speculated that vampirism was caused by a microscopic pathogen. Tragically, he was burned at the stake for heresy, but his research lived on to inspire countless dedicated men and women to bring you the information included on this page.
 |
| HVV carrier: vampire bat |
 |
|
HVV source: the bat flea Ischnopsyllus elongatus |
1. Its capsid bears a distinct bullet shape, although it's about triple the size of rabies, and contains over a thousand more genes.
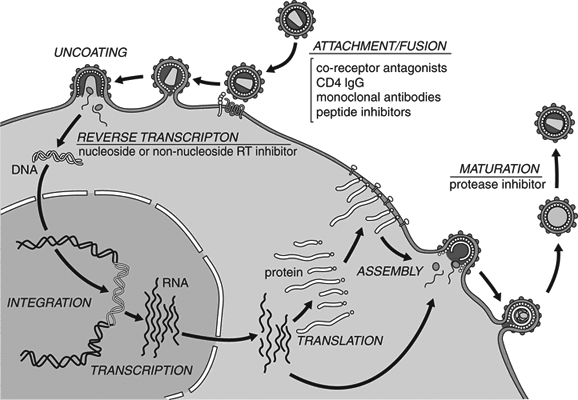
2. It infects cells via endocytosis, meaning that it's absorbed into the cell fully intact. From there, the virus is uncoated so that its genetic material can escape into the cytoplasm. This is different from some other viruses, which either "melt" into the outer membrane or inject their genes from the outside.
3. The virus is zoonotic, meaning that it can be transmitted from one species to another, mainly through biting.
HVV's natural host is a flea commonly found on cave-dwelling bats, including the vampire bat. In the most common scenario, the flea bites a bat, which in-turn passes the virus on to humans and other mammals. It's believed that a vampire's need for blood is derived from both of these animals, possibly through a form of lateral gene transfer between hosts at some point in their evolution.
 |
|
The virus releases its genetic information into the cell, transforming it into a viral factory. |
As touched upon, a virus is a protein shell that introduces its genetic material into the cytoplasm of a vulnerable cell. In effect, the virus takes over the cell's machinery and uses it as a factory for more viral proteins. This usually kills the cell, since it can no longer perform its normal functions, and the release of virus progeny is quite damaging to the cell's membrane. With HVV, the cell is instead altered so that it can "multitask" its own needs—such as regenerating its membrane—while also producing virus clones. Infection of the thyroid gland raises the host's metabolism, allowing the virus to spread even faster throughout the body. This causes gradual weight loss as the body uses up nutrients to fuel the transformation, until the virus enters latency to avoid working the cells to death.
While most viruses are highly specific in what tissues they target, HVV is able to infect every living cell in the body, with the exception of red blood cells. Its replication cycle is also frighteningly rapid, with an incubation period lasting only six to twelve hours.
Although technically a mononegavirus, HVV behaves more like a retrovirus, such as HIV. Once inside a cell's cytoplasm, the virus uses a reverse-transcriptase enzyme to produce DNA from its RNA genome, via the cell's ribosomes. This new DNA is then absorbed into the nucleus and incorporated into the host's genome via an integrase enzyme, becoming a provirus—which in-turn transforms the cell and directs it to synthesize new virions. This is much different from other mononegaviruses, which do not produce DNA or use the nucleus for replication.
While in theory HVV infection is possible through any exchange of bodily fluids, transmission occurs through the bite of an infected person or animal in virtually every case. Provided the immune system is in working order and a proper vaccine is administered in time, the host's body will be able to recognize and destroy any cells showing signs of infection, along with the virions they produce. Depending on the amount of cells destroyed, the patient may require rest and recovery while the body repairs itself. A single vaccination remains effective for up to two years before a booster shot is required—unless of course the victim is drained of blood to the point of reduced immune function.
 |
|
HVV has a wide variety of glycoproteins, which function as keys. |
Locks & Keys: The reason why most viruses can only infect a certain number of tissues is because they're very limited by the amount of genetic information they can carry. This information is needed to construct the "keys" lining the outer capsid, and they have to be compatible with a cell's surface "locks" in order for the virus to infiltrate it. Although every cell has the same DNA, different cell types express different genes, so their outer receptors will be different. There are around 210 types of cells that make up the human body, each with their own locks, and HVV has a special key for almost all of them. Because the virus is so large, it has no trouble storing all the information required to make them.
Antibodies: While it's true that most victims will experience a rapid immune response within several hours of exposure, no actual HVV antibodies are produced before it's too late, since the body has never encountered it before. As you may know, an antibody is a special protein that latches onto a foreign antigen, which allows the immune system to recognize and destroy it. Created by plasma cells, antibodies are unique and work only with the antigens they were made for, and generally aren't produced until days or even weeks after a new infection. HVV symptoms are instead the result of secondary infections and chemical imbalances caused by both the virus and the bite wound it came in through.
Red Blood Cells: Making up approximately one quarter of the 100 trillion cells in the average human body, red blood cells (erythrocytes) are the only ones that lack a nucleus and other organelles. As a result, no virus in existence can infect them, and this includes HVV. However, the virus can still infect the bone marrow, where it can then modify the production of new erythrocytes. Since 2.4 million of them are destroyed and replaced every second, it doesn't take long for much of the bloodstream to change. Still, there are 25 to 30 trillion RBCs in all, and the vampire spleen doesn't work any faster at removing "old" human blood. In effect, it will take a good three to four months for a newly-turned vampire to replace its entire blood volume.
Viral Reactivation: Like the various herpes viruses, HVV usually remains dormant in a fully-transformed vampire—aside from the viral shedding from dying cells into its saliva and other fluids. Only when the immune system is seriously compromised does the virus reawaken and resume its replication cycle, should the following conditions be met: severe malnutrition, extreme psychological stress, and prolonged exposure to ultraviolet rays. Unlike the early replication process prior to post-transformation latency, this form of viral activity is much more damaging, causing shingles-like blisters to erupt from the nerve endings of affected tissues. However, these painful sores will quickly heal if the vampire calms, feeds or gets out of the sun long enough for the virus to become dormant again.